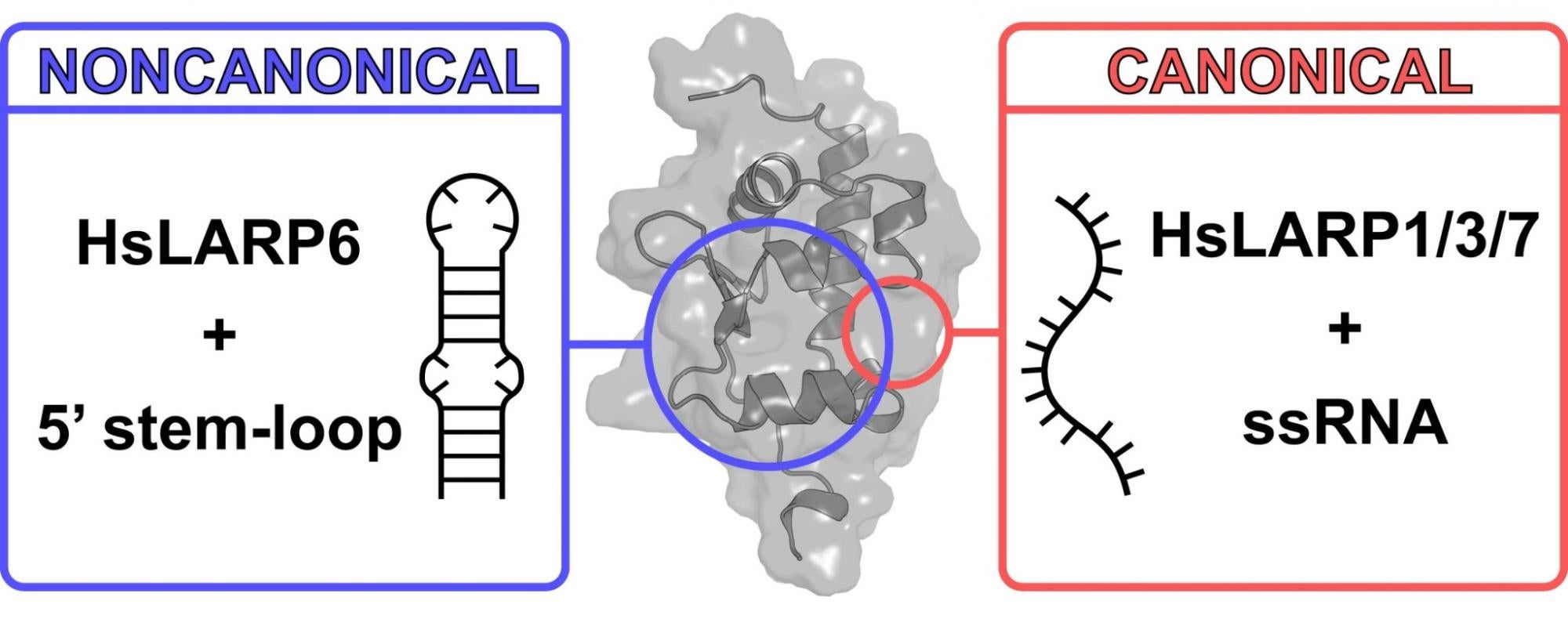
LARPs, or La-related proteins, are a superfamily of proteins common in all plants and animals. They bind RNA, the molecule that carries genetic information, helps build proteins and regulates the function of DNA. LARP6 is one of five main human LARP proteins and is involved in the regulation and biosynthesis of collagen. Compared to other LARPs, there has been very little research into how LARP6 interacts with RNA on a molecular level.
“Our new ‘LEGO piece’ uses a different kind of interaction with its RNA,” said Silvers. “It utilizes a different set of rules and the protein uses a different RNA binding site altogether.”
The team was introduced to this unusual LARP by Branco Stefanovic, a professor in the FSU College of Medicine, who has spent much of his career researching fibrosis. They attempted multiple methods of observing the protein, such as X-ray crystallography, before landing on NMR spectroscopy.
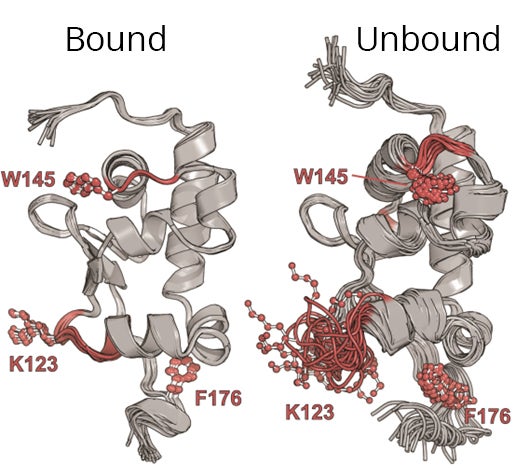
“In NMR spectroscopy, we can look at the complex in solution close to its natural environment under physiological conditions,” said Silvers. “NMR spectroscopy is ideal as we can study the dynamics of a molecule as well as its structure.”
NMR spectroscopy is a method that uses the magnetic properties of certain nuclei to reveal a molecule’s detailed structure and properties. It proved especially useful because LARP6 is unstable until it binds with RNA.
Using NMR spectroscopy, the researchers found that the way LARP6 binds to RNA is directly involved in the biosynthesis of type I collagen, the protein involved in fibrosis. This discovery could help scientists develop a treatment for fibrosis in the future.
“Because of its function, the complex between LARP6 and RNA is something that we potentially can develop a drug for, to work against fibrosis,” said Silvers. “There is currently no drug, to my knowledge, that can slow down or stop the progression of fibrosis.”
This research was funded by the National Institutes of Health.